Cluster of differentiation (CD) marker panel 28th October 2021 – Tags: CD, cd marker, Cluster of differentiation, Cluster of differentiation (CD), marker panel
Introduction
In this article we will talk in depth about the Cluster of Differentiation (CD) marker panel, CD is short for “Cluster of Differentiation”. CD molecules are cell surface markers that are very useful for the identification and characterisation of the cell surface. leucocitos and of the different leukocyte subpopulations. The HLDA (Human Leukocyte Differentiation Antigens) workshop, which started in 1982, developed the CD nomenclature and has maintained the list of CD markers ever since. The initial idea of the CD nomenclature was the classification of many different monoclonal antibodies against leukocyte cell surface molecules that had been generated by different laboratories around the world.
The number of CD markers has grown steadily and has been extended to other cell types. Today there are more than 320 DC groups described in humans.
CD marker - mAbs against leukocyte surface molecules
Cluster of Differentiation (DC) markers are particularly useful for the identification of the leukocyte population by flow cytometry. Our resource pages General flow cytometry protocol and What is flow cytometry? provide a brief introduction to flow cytometry.
The great advantage of flow cytometry is that it allows the simultaneous detection of several markers in a single cell at the same time. With a modern flow cytometer, 8 to 10 different colours can easily be measured in a sample, and the most advanced cytometers can even measure up to 18 channels at a time.
Determining leukocyte populations with CD markers
When flow cytometry is used to determine a particular leukocyte population, a combination of several Cluster of Differentiation markers is often used to determine a subpopulation, as a single marker does not usually define the entire population.
Typically, researchers generate gating trees when evaluating flow cytometry experiments.
A very illustrative example is the distribution of CD8. Although CD8 is the most prominent marker and namesake of CD8-positive cytotoxic T cells (CD8+), it is also expressed by natural killer cells (NK cells) and dentritic cells (DCs). If all CD8+ cells are separated from a leukocyte population in a blood sample, the separated population would consist of CD8+ T cells, NK cells and Cumulus Differentiation Cells (DC).
In the following experiment, the ratio of CD4+ T helper cells to CD8+ cytotoxic T cells was determined. First, all CD45+ cells (general leukocyte marker) were separated from the peripheral blood sample (gate 1).
In the following panel, all CD45+ cells in Gate 1 were blotted to determine their CD3 expression. CD3 is the most common T-cell marker, which is only expressed on T cells and not on other CD8+ cells, such as NK or DC cells. The selection of CD3+ cells ensures that CD3- cells are not counted as CD8+ T cells but CD8+ cells (e.g. NK or DC cells).
The CD3+ T cell population can further differentiate into cytotoxic CD8+ T cells and CD4+ T helper cells, as shown in panel 2, where CD3+ cells from gate 2 were blotted for CD4 and CD8 expression. CD4+ CD8- helper T cells are in the upper left quadrant and CD4- CD8+ cytotoxic T cells are shown in the lower right quadrant of panel 3.
If necessary, a researcher interested in different T helper populations, such as TH1, TH2, TH9, TH17 and Treg, would stain the respective extra- or intracellular markers of these subpopulations.
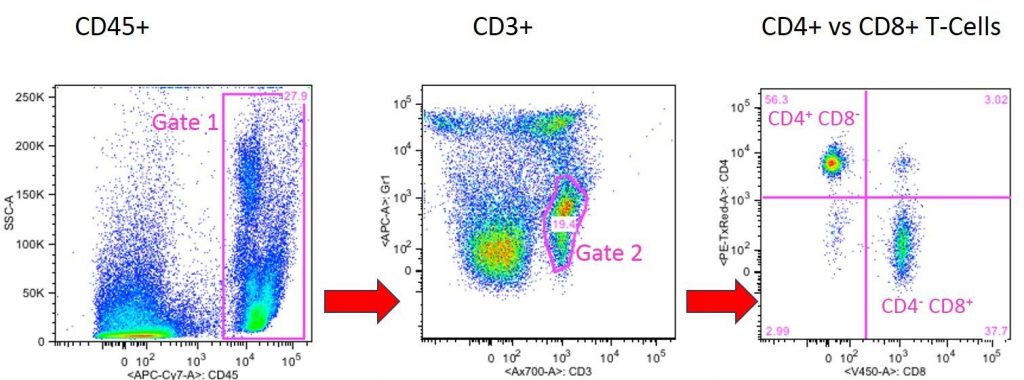
Example of how CD markers are used to define cell populations in flow cytometry. The image shows the Gating tree of CD4+ and CD8+ T cells. In the left panel, leukocytes (CD45+ cells) are grouped in gate 1. In the middle panel, only gate 1 cells are shown, which are all the leukocytes in the sample. In this panel the T lymphocytes (CD3+) are gated in gate 2. The right panel shows CD3+ T cells and separates CD4+ and CD8- T Helper cells (upper left quadrant) and C8+ and Cd4- cytotoxic cells (lower right panel). Courtesy of Dr. F.M. Hess, Boisvert Lab, JABSOM, University of Hawaii.
You can continue reading in our blog

